540

Reģistrācijas numurs: 50203081351
- Apraksts
- Pamatinformācija
- Uzņēmums
Qualifications and Experience:
- Bachelors or Masters degree in Chemical Engineering, Biochemistry, Biotechnology, Industrial Engineering, or a related field.
- Minimum 5 years of experience in reagent manufacturing, bioprocessing, or related production consulting roles.
- Strong understanding of GMP, ISO 13485, and regulatory requirements in a life sciences or diagnostics environment.
- Experience with process validation, equipment qualification, and risk management.
- Proficient in Lean Manufacturing, Six Sigma, or similar methodologies (certification a plus).
- Skilled in documentation systems (e.g., ERP, PLM, QMS) and production data analysis.
- Excellent communication, training, and cross-functional collaboration skills.
Preferred Qualifications:
- Hands-on experience with cleanroom operations and aseptic processing.
- Experience scaling production from pilot to commercial stages.
- Familiarity with laboratory automation and digital manufacturing tools.
Key Responsibilities:
Job Purpose: This position is responsible for evaluating current production workflows, identifying inefficiencies, and recommending improvements in line with GMP standards, lean manufacturing principles, and regulatory compliance.
Process Assessment and Optimization
- Analyse existing reagent production processes
- Recommend and implement process improvements
Workflow Standardization
- Develop or refine SOPs, Work Instructions, and batch records to standardize production practices.
- Ensure alignment with GMP, ISO standards, and other regulatory requirements.
Technology and Equipment Recommendations
- Assess current production technologies and equipment capabilities
- Recommend upgrades or new tools to increase automation
Data-Driven Decision Making
- Use production data, KPIs, and root cause analysis tools to guide process improvement decisions
- Implement control measures and monitoring systems
Cross-Functional Collaboration
- Work closely with Manufacturing, Quality Assurance, Engineering, and R&D teams to align improvements with business goals and product requirements.
Training and Support
- Provide training and mentoring to production teams on new or revised procedures.
Lean and Continuous Improvement
- Lead or contribute to Lean, Kaizen, or Six Sigma initiatives
Compliance and Validation
- Ensure all process changes are documented and validated according to QMS procedures.
We Offer:
- Health Insurance Policy - keeping you covered and well.
- Monthly salary EUR gross in a range 1100 - 1600.
- Enjoy co-paid lunch option available at the office for your convenience.
- Teambuilding Events - opportunities to connect, engage, and grow with colleagues.
- Energy-Efficient Office Environment - work in a modern, sustainable facility that promotes environmental responsibility.
- Free Onsite Car Parking - convenient and hassle-free.
- Culturally Diverse, Multinational Team - join a workplace that values inclusion and collaboration across cultures.
GDPR Privacy Notice:
Data Controller: Latvia MGI-Tech, Lidostas parks, Māruoe
When you apply to Latvia MGI-Tech we are committed to protecting your personal data in accordance with the General Data Protection Regulation (GDPR). By submitting your application, you agree to the collection, processing, and storage of your personal information by Latvia MGI-Tech for recruitment purposes.
What Data We Collect and Why
We collect and process personal information that you provide as part of your application, including but not limited to your name, contact details, work experience, and education history. This information is necessary to assess your application and contact you regarding this recruitment process.
Data Retention
Your personal data will be stored only for the duration necessary to complete the recruitment process for the position you applied for. If your application is unsuccessful, Latvia MGI-Tech will not retain your data.
Data Sharing
Your information will be processed internally within Latvia MGI-Tech. We do not share your information with third parties.
Your Rights
Under the GDPR, you have the right to access, rectify, or erase your personal data, and to restrict or object to its processing. To exercise these rights or if you have questions regarding our data protection practices, please contact our Data Protection Officer.
Consent
By submitting your application, you consent to the collection and processing of your personal data in line with this notice. Please do not include any sensitive personal information (such as health or financial information) unless it is relevant to the job requirements.
€
2000 - 3000
Papildu informācija: Health insurance after probation period, annual bonus.
Atrašanās vieta
- Mārupe, Mārupes novads, Latvija
Lidostas parks Mārupes pagasts LV-2167
Darba veids
- Pilna slodze
Valodas
- Angļu
Kontaktpersona
Sandra Vilsone
Sandra Vilsone
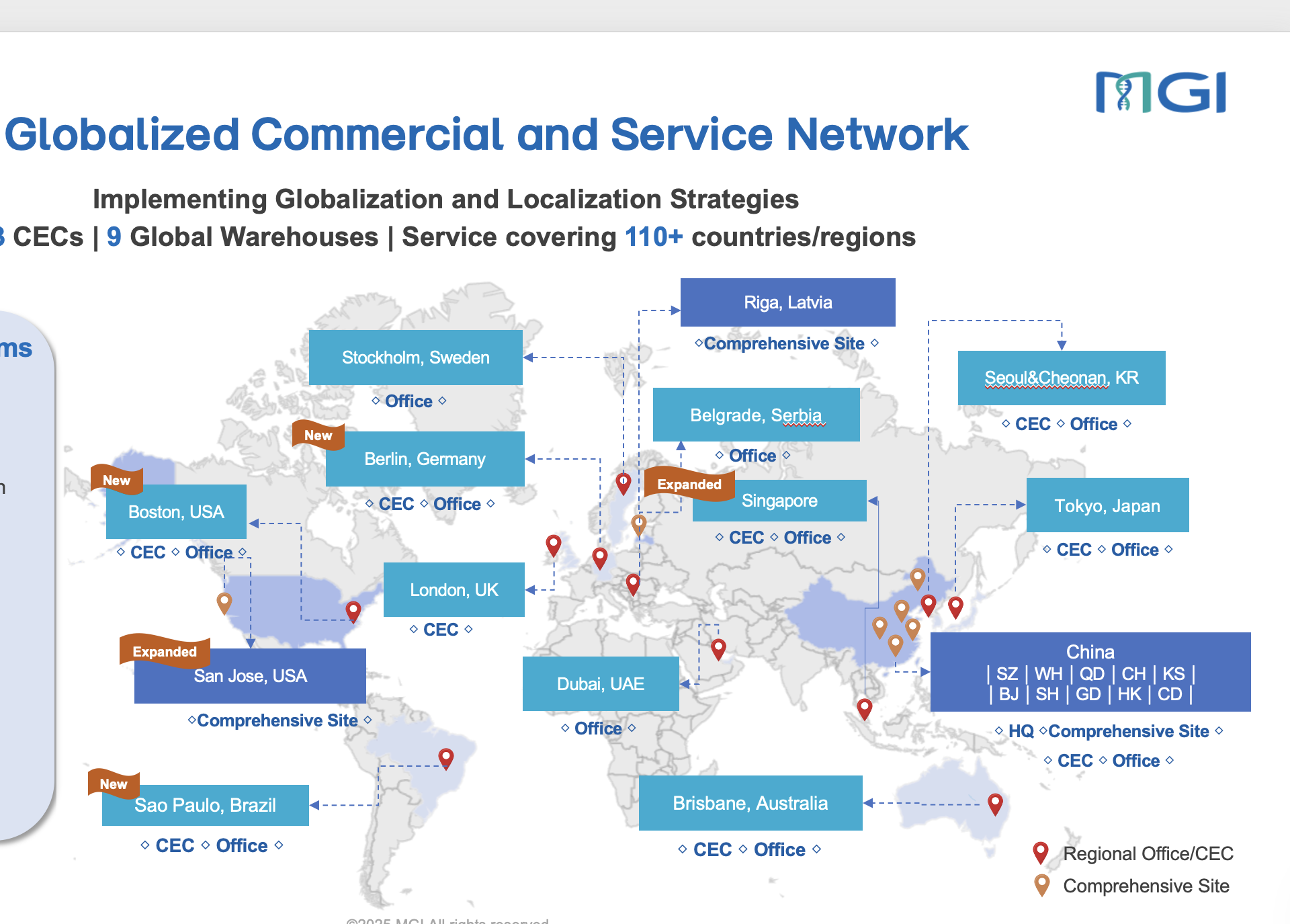
About MGI
MGI Tech Co., Ltd. (MGI), a member of global genomics leader BGI Group, is committed to building core tools and technology to lead life science through intelligent innovation. Based on its proprietary technology, MGI focuses on R&D, production and sales of sequencing instruments, reagents, and related products to support life science research, agriculture, precision medicine and healthcare. MGI is a leading producer of clinical high-throughput gene sequencers, and its multi-omics platforms include genetic sequencing, mass spectrometry, medical imaging and laboratory automation.
Reģistrācijas numurs: 50203081351
Tev varētu interesēt arī:
Pārdošanas Speciālists
E-commerce and after sales specialist
Uzņēmuma izpilddirektors/-e